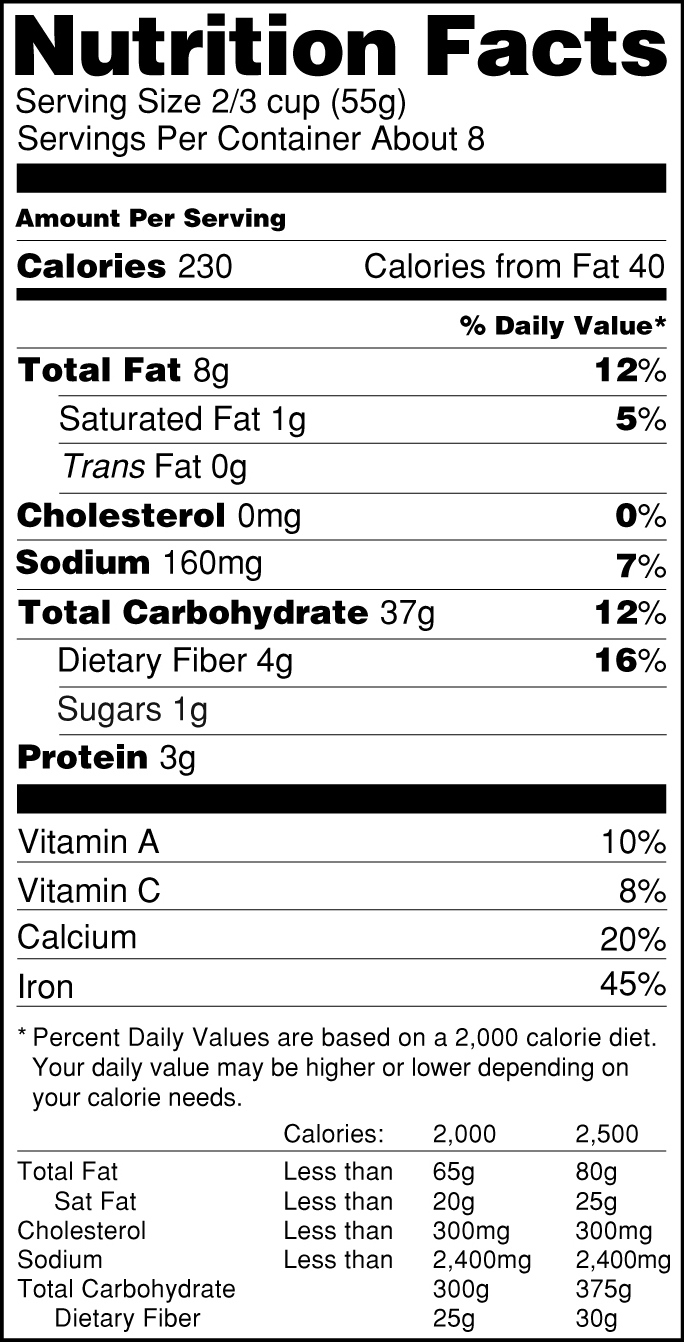
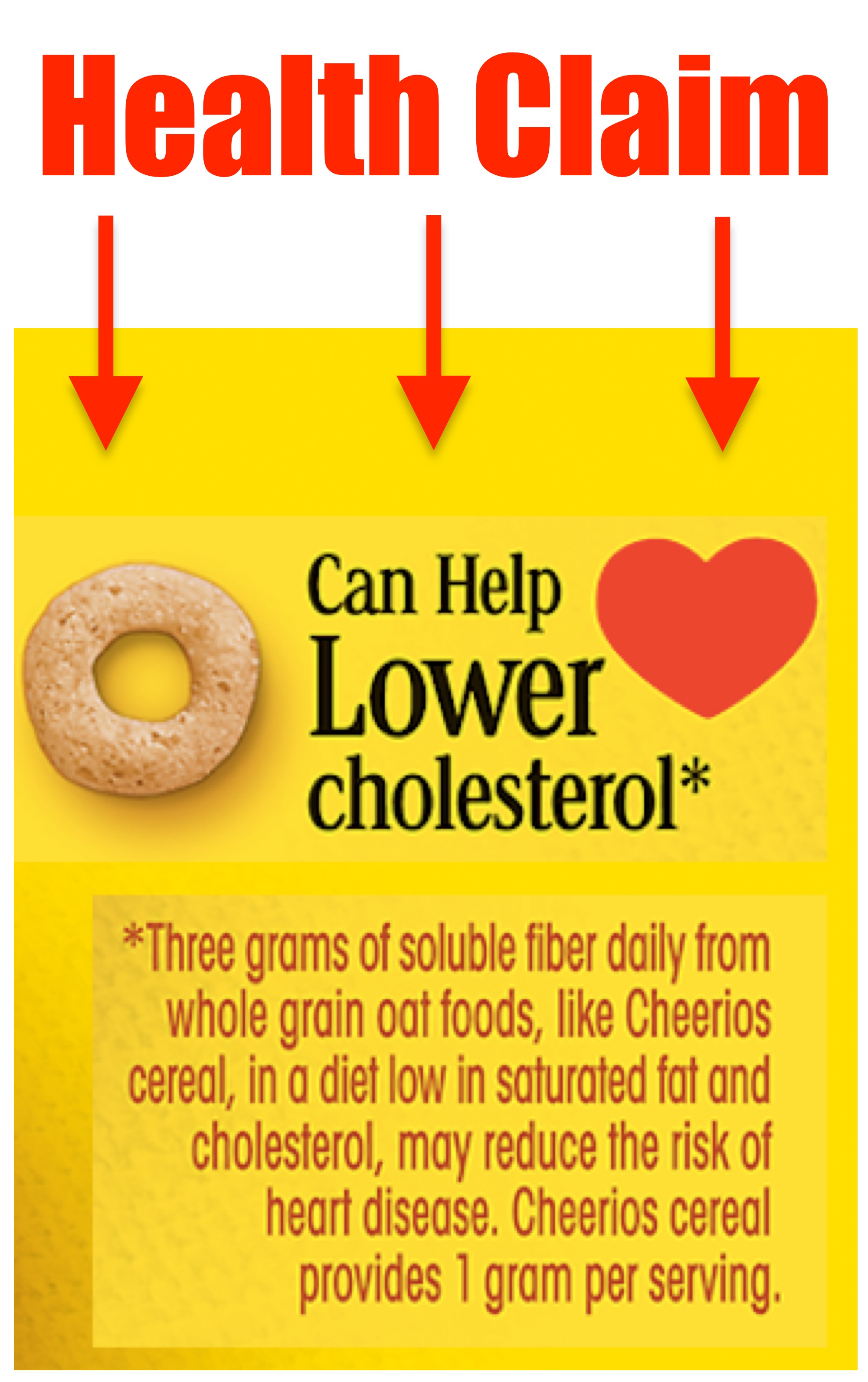
40 health claims on food labels are standardized and regulated
Dietary supplement - Wikipedia Definition. In the United States, the Dietary Supplement Health and Education Act of 1994 provides this description: "The Dietary Supplement Health and Education Act of 1994 (DSHEA) defines the term "dietary supplement" to mean a product (other than tobacco) intended to supplement the diet that bears or contains one or more of the following dietary ingredients: a vitamin, a mineral, an herb or ... Trans fat - Wikipedia Trans fat, also called trans-unsaturated fatty acids, or trans fatty acids, is a type of unsaturated fat that naturally occurs in small amounts in meat and milk fat. It became widely produced as an unintentional byproduct in the industrial processing of vegetable and fish oils in the early 20th century for use in margarine and later also in snack food, packaged baked goods, and for frying fast ...
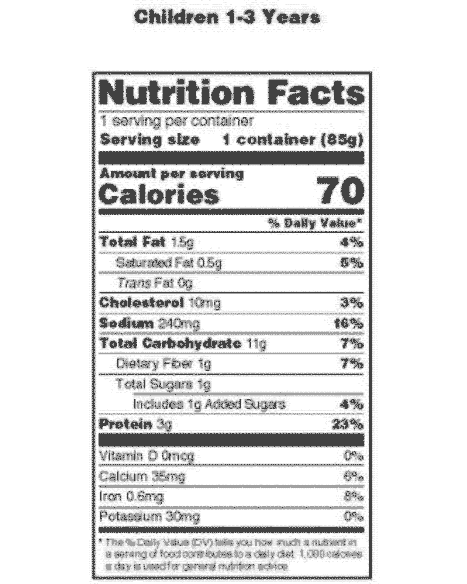
Vitamin D - Health Professional Fact Sheet The labels must list vitamin D content in mcg per serving and have the option of also listing the amount in IUs in parentheses. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet. ** Vitamin D is in the yolk.
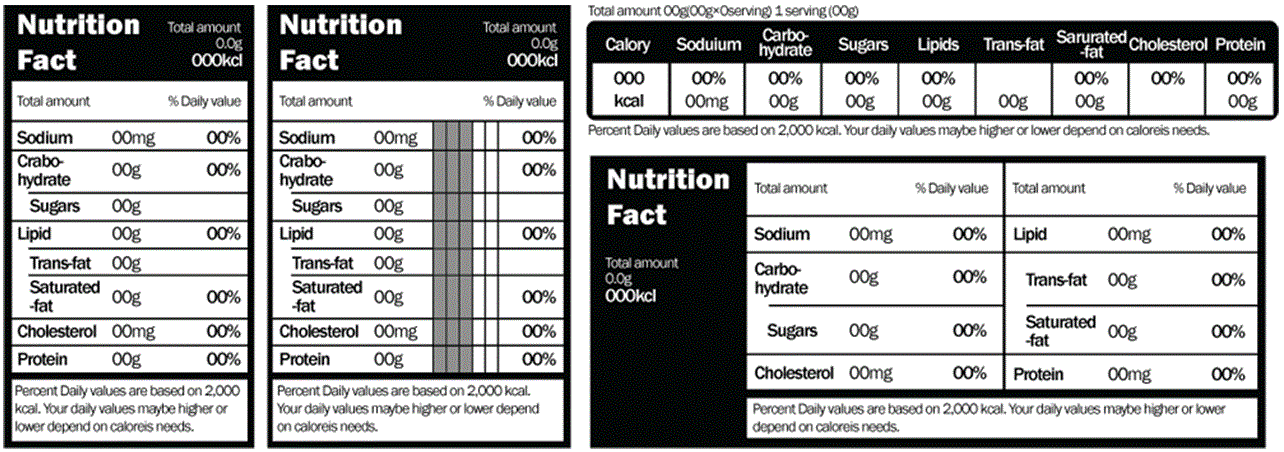
Health claims on food labels are standardized and regulated
Labeling & Nutrition Guidance Documents & Regulatory ... Interim Procedures for Qualified Health Claims in the Labeling of Conventional Human Food and Human Dietary Supplements (July 2003 FDA's Implementation of "Qualified Health Claims": Questions and ... Guidelines for the Safety Assessment of Novel Foods At present, the Food and Drug Regulations permit only certain health and nutrition claims for foods, i.e. those referred to in section B.01.311 ("biological role claims"), tables following section B.01.513 (nutrient content claims), and section B.01.603 (health claims). Food/Dietary Supplement Guidance and Regulatory Information Sep 30, 2022 · 2014. WITHDRAWN Acidified and Low-Acid Canned Foods: (DRAFT) Submitting Form FDA 2541 (Food Canning Establishment Registration) and Forms FDA 2541d, FDA 2541e, FDA 2541f, and FDA 2541g (Food ...
Health claims on food labels are standardized and regulated. Prostate Cancer, Nutrition, and Dietary Supplements (PDQ ... Companies distribute calcium as a dietary supplement. In the United States, dietary supplements are regulated as foods, not drugs. Therefore, premarket evaluation and approval of such supplements by the U.S. Food and Drug Administration (FDA) are not required unless specific disease prevention or treatment claims are made. The FDA can remove ... Food/Dietary Supplement Guidance and Regulatory Information Sep 30, 2022 · 2014. WITHDRAWN Acidified and Low-Acid Canned Foods: (DRAFT) Submitting Form FDA 2541 (Food Canning Establishment Registration) and Forms FDA 2541d, FDA 2541e, FDA 2541f, and FDA 2541g (Food ... Guidelines for the Safety Assessment of Novel Foods At present, the Food and Drug Regulations permit only certain health and nutrition claims for foods, i.e. those referred to in section B.01.311 ("biological role claims"), tables following section B.01.513 (nutrient content claims), and section B.01.603 (health claims). Labeling & Nutrition Guidance Documents & Regulatory ... Interim Procedures for Qualified Health Claims in the Labeling of Conventional Human Food and Human Dietary Supplements (July 2003 FDA's Implementation of "Qualified Health Claims": Questions and ...















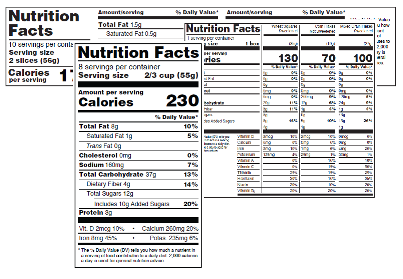



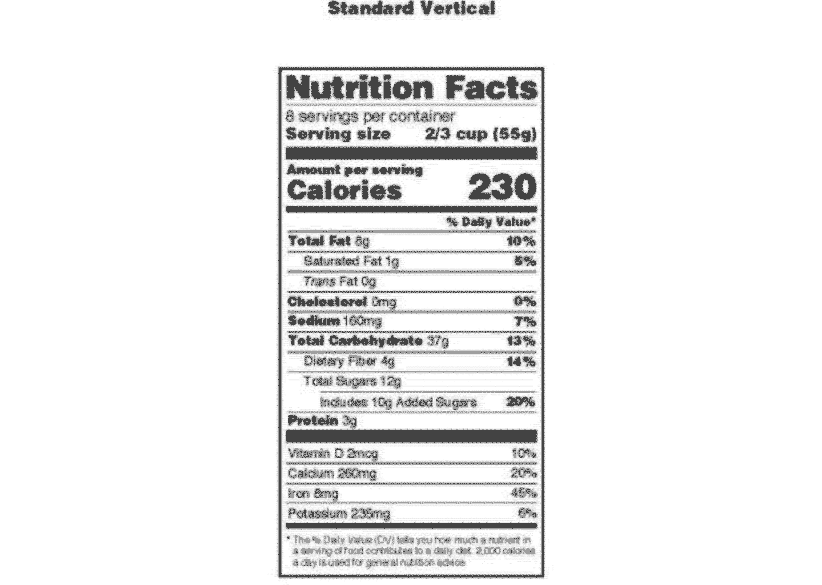












Post a Comment for "40 health claims on food labels are standardized and regulated"